Influenza threat is one that keeps evolving every year rendering vaccines ineffective and completely useless – something that researchers have long been trying to address.
In a new research that has upped the about a hope for a more effective, long-lasting flu vaccine, scientists at The Scripps Research Institute (TSRI) and the Janssen Pharmaceutical Companies of Johnson & Johnson (Janssen) have come up with a new method using which antibodies can be induced to fight a wide range of influenza subtypes, thereby eliminating the need for seasonal flu shots.
Statistics reveal that in the US alone, seasonal flu causes 36,000 deaths annually while over 200,000 are hospitalised. Doctors have been recommending yearly flu shots, but these vaccines are not able to provide the desired protection against all flu subtypes and because the strain keeps evolving, these shots are almost useless in a few months time.
Researchers at TSRI, Janssen and other institutions have shown that some people are capable of making powerful antibodies that can fight many subtypes of influenza at once by targeting a site on the influenza virus that does not mutate rapidly. Unfortunately, these “broadly neutralizing antibodies,” or bnAbs, are rare.
Still, the tantalizing existence of broadly neutralizing antibodies led Janssen and TRSI to try creating an influenza vaccine specially designed to elicit them.
Studies and experiments allowed researchers to zero in on a possible target: a protein on the surface of influenza, called hemagglutinin (HA). HA is present on all subtypes of influenza, providing the key viral “machinery” that enables the virus to enter cells. Most importantly, the long “stem” region of HA, which connects the virus to cells, plays such a crucial role that mutations at the site are unlikely to be passed on.
“If the body can make an immune response against the HA stem, it’s difficult for the virus to escape,” Wilson explained.
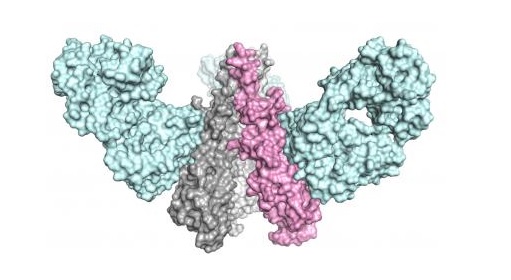
The team from The Scripps Research Institute and Janssen Pharmaceutical Companies designed a molecule that mimicked the shape of a key part of the influenza virus, inducing a powerful and broadly effective immune response in animal models. Image courtesy of the Wilson lab, The Scripps Research Institute.
This vaccine candidate was designed, produced and tested by a team of scientists led by Jaap Goudsmit, head of the Janssen Prevention Center, the paper’s first author Antonietta Impagliazzo (responsible for the design) and co-senior author Katarina Radoševi.
Scientists carried out studies on rodent and nonhuman primate models to check out the response. They found that animals given one especially stable immunogen produced antibodies that could bind with HAs in many influenza subtypes, even neutralizing H5N1 viruses (“avian” or “bird” flu).
“This was the proof of principle,” said Ian Wilson, Hansen Professor of Structural Biology and chair of the Department of Integrative Structural and Computational Biology at TSRI. “These tests showed that antibodies elicited against one influenza subtype could protect against a different subtype.”
Scientists at TSRI studied the structure of the immunogen at every point in the process. Using the imaging techniques of electron microscopy (led by TSRI Associate Professor Andrew Ward and postdoctoral fellow Ryan Hoffman) and x-ray crystallography (led by Wilson and TSRI Staff Scientist Xueyong Zhu), the team showed that the most promising candidate immunogen mimicked the HA stem and that antibodies could bind with the immunogen just as they would with a real virus.
With proof that an immunogen can elicit antibodies against the stem region, Wilson said the next step in this research is to see if the immunogen can do the same in humans.
“While there is more work to be done, the ultimate goal, of course, would be to create a life-long vaccine,” Wilson said.
The research is published in the journal Science.
